Research
Total Synthesis of Natural Products, Heterocyclic and Medicinal Chemistry, and New Synthetic Methods. We also pursue projects in catalysis, flow-, and photochemistry, and we explore novel concepts in Chemical Biology. Group Members develop unique expertise in small molecule synthesis (tactics), problem solving (strategies), and interdisciplinary research (collaborations).
Selected Publications
Small Molecule Inhibitors of Protein Kinase D: Early Development, Current Approaches, and Future Directions.
Quiming Jane Wang, Peter Wipf. J. Med. Chem. 2023, 66, 122.
Now entering its fourth decade, research on the biological function, small molecule inhibition, and disease relevance of the three known isoforms of protein kinase D, PKD1, PKD2, and PKD3, has entered a mature development stage. This mini-perspective focuses on the medicinal chemistry that provided a structurally diverse set of mainly active site inhibitors, which, for a brief time period, moved through preclinical development stages but have yet to be tested in clinical trials. In particular, between 2006 and 2012, a rapid expansion of synthetic efforts led to several moderately to highly PKD-selective chemotypes but did not yet achieve PKD subtype selectivity or resolve general toxicity and pharmacokinetic challenges. In addition to cancer, other unresolved medical needs in cardiovascular, inflammatory, and metabolic diseases would, however, benefit from a renewed focus on potent and selective PKD modulators.
Link.
C3-Functionalization of Indoles with alpha-Heteroaryl-Substituted Methyl Alcohols.
Ethan Pazur, Nikhil R. Tasker, Peter Wipf. Org. Biomol. Chem. 2023, 21, 8651.
The transition metal-free Cs2CO3/Oxone®-mediated C3-alkylation of indoles proceeds in moderate to high yields with a variety of C4–C7 functionalized indoles and is applicable to 2-, 3- and 4-hydroxymethyl pyridines and related electron-deficient heterocycles, permitting novel late-stage drug functionalizations. Preliminary mechanistic studies support a hydrogen autotransfer-type chain process starting with an initial oxidation of the alcohol to the corresponding aldehyde, followed by a subsequent condensation onto indole and reduction/hydride delivery from another equivalent of the primary alcohol.
Link.
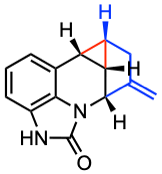
Matteo Borgini, Peter Wipf. ChemRxiv 2023.
Bicyclo[1.1.0]butane (BCB)-containing compounds are a highly strained class of reagents that feature a unique chemical reactivity, trig-gering “strain-release” reaction cascades, and provide unique products with considerable utility in the drug discovery field. The C-C bridgehead bond of BCB’s two fused cyclopropane rings holds significant π-character, allowing BCB-containing compounds to react with nucleophiles, electrophiles, radicals, π-systems, and carbenes. Herein, we reported the synthesis of new BCBs by the trapping of nu-cleophilic intermediates with quaternary ammonium ions derived from quinolines and pyridines. The resulting BCBs are then converted with high regioselectivity to unprecedented fused heterocycles in a rhodium(I)-catalyzed annulative rearrangement.
Link.

Biosynthesis, Total Synthesis, and Biological Profiles of Ergot Alkaloids.
Nikhil R. Tasker, Peter Wipf. The Alkaloids. 2021, 85, 1.
While the use of ergot alkaloids in folk medicine has been practiced for millennia, systematic investigations on their therapeutic potential began about 100 years ago. Subsequently, Albert Hofmann's discovery of lysergic acid diethylamide (LSD) and its intense psychedelic properties garnered worldwide attention and prompted further studies of this compound class. As a result, several natural ergot alkaloids were discovered and unnatural analogs were synthesized, and some were used to treat an array of maladies, including Alzheimer's and Parkinson's disease. While LSD was never commercially approved, recent clinical studies have found it can be an innovative and effective treatment option for several psychiatric disorders. Ongoing biosynthetic and total synthetic investigations aim to understand the natural origins of ergot alkaloids, help develop facile means to produce these natural products and enable their continued use as medicinal chemistry lead structures.
https://doi.org/10.1016/bs.alkal.2020.08.001.
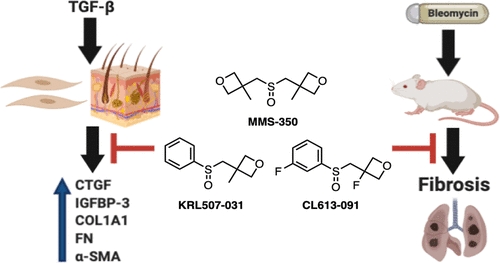
Oxetanyl Sulfoxide MMS-350 Ameliorates Pulmonary Fibrosis In Vitro, In Vivo, and Ex Vivo.
Logan Mlakar, Jessica Lane, Takahisa Takihara, Chaemin Lim, Melissa M. Sprachman, Kayla R. Lloyd, Peter Wipf, Carol Feghali-Bostwick. ACS. Med. Chem. Lett. 2020,11, 2312.
Fibrosis is a common feature of several diseases, involves different organs, and results in significant morbidity and mortality. There are currently no effective therapies to halt the progression of fibrosis or reverse it. We have identified the highly water-soluble MMS-350, a novel bis-oxetanyl sulfoxide, as an antifibrotic agent. MMS-350 reduced the profibrotic phenotype induced in vitro in primary human fibroblasts and ameliorated bleomycin-induced pulmonary fibrosis in vivo. Furthermore, MMS-350 reversed fibrosis in human skin in organ culture. MMS-350 reduced levels of extracellular matrix proteins, the activation of fibroblasts, and the induction of pro-fibrotic factors. Similar effects at lower concentrations were observed with KRL507-031 and CL-613-091, two more lipophilic MMS-350 analogues. The fact that MMS-350 was effective at reducing pulmonary fibrosis induced by different triggers, the differential biological effects of its close structural analogues and its oral availability make it an attractive therapeutic candidate for organ fibrosis.
https://doi.org/10.1021/acsmedchemlett.0c00433".
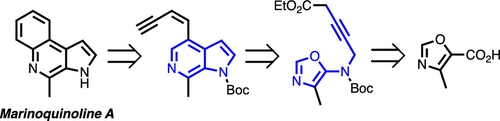
Mana Osano, Dhishit Jhaveri, Peter Wipf. Org. Lett. 2020, 22, 2215.
A new variant of the intramolecular Diels–Alder oxazole (IMDAO) cycloaddition that provides direct access to 6-azaindoles was developed. The IMDAO reaction was applied in a total synthesis of the aminophenylpyrrole-derived alkaloid marinoquinoline A, also featuring the use of a Curtius reaction for preparation of a 5-aminooxazole, a propargylic C,H-bond insertion, an in situ alkyne–allene isomerization, and a ruthenium-catalyzed cycloisomerization for benzene ring annulation to the 6-azaindole.
Bibliography Links
In addition to SciFinder, our research abstracts and publication records can be accessed through a variety of databases, including:
A brief overview of our main research interests and pertinent recent papers is also available here.