

Wipf Group - Good & Required Laboratory Practices
- First and foremost, it is imperative that lab notebooks are kept in accordance to ACS guidelines and the standard of good laboratory practices. Have a look at this web link , a summary I wrote for an undergrad lab course, and the sample notebook pages (the good, the bad, and the ugly)! Also, make sure you use the appropriate number of significant figures in your experimental part. The ACS "Writing the Laboratory Notebook" is on file in our Group Collection. For an adequate experimental progress report, see here.
Good laboratory practice is strongly dependent on reliable, current, and detailed records in your lab e-notebook. All quantitative measurements have to be clearly noted, and full, detailed sentences must be used to describe the course of the reaction. Your experimental part must be readable to easy to reproduce! Write a draft of your reaction plan before starting the experiment, print out the e-notebook page and take it to the hood, update it during the experiment when you make important observations or changes in the planned procedure (such as reagent weights and reaction times), and finalize the e-notebook entry immediately after you finish the reaction. Measured amounts of reagents and dried products, and yield, need to be listed in the Procedures (and not just in the ELN calculation spreadsheet). TLC or other analytical measurements (NMR, MS, mp, [a]D, IR, etc) must be recorded accurately, including the solvent and the assignment of reference samples.
Keeping a valid, up-to-date e-notebook is a critical feature of your practical training and your professional career. For every reaction, a new e-notebook page with a detailed experimental protocol has to be created before the reaction is started, even if it is a repeat, scale-up, or duplicate of a previous reaction. Detailed experimental procedures, including order of addition of reagents, reaction times, specific workup protocols, and all supporting data, such as physical observations, product weights and yields, TLCs and available LCMS data, have to be uploaded daily, usually at the end of the day or maybe at the beginning of the next day. The e-notebook page has to be approved as soon as the spectroscopic data collection is complete, usually within 1 week, but certainly no later than 2 weeks after the experiment. Any original hardcopies (of spectra, etc) have to be kept in the lab at all times, and not anywhere else. The e-notebook always has to be kept up-to-date on all current experiments. If a procedure, reaction or compound is not present as an accurate entry in your e-notebook, it does not "count" and cannot be reported in group meetings, presentations, or paper drafts. The e-notebook is the only authentic notebook; loose pages or unrecorded "drafts" are not permissible. You can not modify an experimental part in a paper or thesis draft by deviating from the data listed in your e-notebook.
••••• Information about our new electronic inventory and notebook system •••••
It is crucial that everybody realizes that for every compound that you claim to have synthesized you need full experimental characterization, which includes Mp (if a solid), [a]D (if chiral and not racemic), IR, 1H NMR, 13C NMR, MS and HRMS. The 1H and 13C NMR spectra have to be clean, since they serve as criteria for compound purity in the absence of elemental analyses, which we usually don’t get. Therefore, solvent peaks or other impurities, known or unknown, are not acceptable. Since we generally submit copies of spectra to ACS journals as supplementary material, 1H and 13C NMR spectra have to be of high quality and in letter size format with clearly readable labels. In the context of a longer synthesis, you can use your judgment and partially characterize some intermediates, but fully characterize major intermediates and the final compounds, yet you should not go for more than three steps without full characterization. You will be unable to publish or submit your candidacy/MS/PhD thesis/paper if your samples are not fully characterized. With your thesis, please include a compound characterization checksheet (JOC format) for all compounds, and include copies of 1H and 13C NMRs of key compounds in your hardcopy.
I urge you to be conscientious in writing up experimental procedures and characterizing your compounds diligently. Delaying full characterization, an ELN with incomplete or incorrect entries (or none at all), and sloppy record keeping are THE recipe for wasted efforts and failure.
Only accurate and honestly generated and reported data are acceptable!! If there are any questions about the validity of a claim, or the integrity of an experiment, voice them immediately and do not use the data in any form, be it a research report, a conference proceeding, or a thesis or paper draft. Several web sites provide detailed information about University and Departmental Ethical Research Conduct guidelines, including plagiarism, misuse of privileged information, data integrity, and obligation to report.
It is significant to consider the serious consequences of misconduct; this document summarizes the University of Pittsburgh policies on Research Integrity, but beyond all the legal wording, be aware of all the damage scientific misconduct will cause for you, your colleagues, and everybody affiliated with our group and our Department.
The CITI/ University of Pittsburgh training module "Responsible Conduct of Research" needs to be completed by all Wipf Group members. Please turn in a signed copy of the completion record for our files.
- Writing your Thesis/Paper: Have a look at our publications and experimental data/supplementary material posted on the ACS journals webpages. Also, consult the "ACS Style Guide" which is on file in our Group Collection. At any given time, several of you are in the process of writing a thesis, report, or a paper draft. In addition to devising an outline that raises the interest of the reader, rather than putting him or her to sleep, making sure that all the experimental data are presented and corrected (yes, proofread yourself several times and have somebody else proofread for you!), and applying logical reasoning, make sure that you give proper credit for any “borrowed” material!
Have a look at Ashlyn's recommendations for the Candidacy Exam.
- Avoid plagiarism! Scholarship builds on the knowledge, understanding, and creative extrapolation of the work of others, but there are strict rules for ethical scholarship and for avoding plegiarism. In this link, I have summarized a few important guidelines from the ethics websites of the University of Pittsburgh and the University of California. Please adhere strictly to these rules; otherwise you will face at least a significant delay in the correction of your work and at worst a failure in an exam (or annulation of your degree). Keep in mind that you have to avoid even self-plagiarism. Let me know if there are any questions!
- Purity of Compounds. All new compounds, whether synthesized or purchased, should normally possess a purity of at least 95%. All scientifically established methods (e.g., HPLC, LC/MS, combustion analysis) of establishing purity are acceptable. The specific analytical method used to determine purity should be included in the general part of the experimental section together with a statement confirming ≥95% purity. The experimental data supporting this statement should be uploaded in your CBIS ELN pages, but no further documentation is required for publication in an ACS journal, a formal report, or a thesis, unless specifically required. Spectral data is often not sufficient as a criterion of purity. When the purity of a particular compound is less than 95%, the percent of purity should be specified at the end of the description of its synthesis in the experimental section. If the compounds contain solvent, the quantity of solvent should be included in the experimental description.
- Guidelines for Sample Handling for NMR Analyses and Biological Assay Submissions: We generally only submit samples that pass our internal QC (LCMS/ELSD) for 90-95% purity. Sometimes, impurities are introduced due to inappropriate sample handling. Please check this SOP for some typical problems and solutions. CDCl3 fresh from the bottle (new, old, same problem) contains significant amounts of HCl that can decompose samples within minutes. Any acid-sensitive samples should only be dissolved in CDCl3 that has been freshly filtered through basic alumina (Al2O3) (use a pipette filter) - usually 0.5 mL is the appropriate amount of deuterated solvent to dissolve your sample. For the same reason, it is never a good idea to dump your NMR sample back into the flask containing your bulk material.
- Chemical Inventory: In order to allow efficient access to group chemicals, please give the package slip of any chemical that you receive to Courtney. The Group Member(s) in charge of the inventory will add this information into our database, which is updated on a monthly basis. Also, please let Courtney know when you use up a chemical, so that the entry can be deleted from the index!
- Safety! Please check our webpage for general safety information. Inform yourselves about the hazards of any new chemical before using it! If you are not sure about any potential hazard, check with me first. The best safety net is a well-prepared and thoughtful experimental protocol - never rush into an experiment!
- Clean, Orderly Hoods reflect good experimental techniques! Your hood and bench should reflect professionalism, efficiency, and appreciation for a safe working environment. This real-life example does clearly not meet the minimally acceptable standards:

Also, common areas are everybody's responsibility and should be kept clean- not as shown here:

- THINK AHEAD of what could go wrong when you set up your reaction! The following two pictures show at least three clear violations of professional standards for how to heat a reaction at reflux. Not surprisingly, this faulty setup caused a major flood minutes after the start of the reaction, but was fortunately discovered by more observant lab mates while the aspiring scientist had already left the lab... Always watch your reaction until the conditions have stabilized, the oil bath has reached its set temperature, the reflux is consistent, or the exothermic process has stopped, etc.
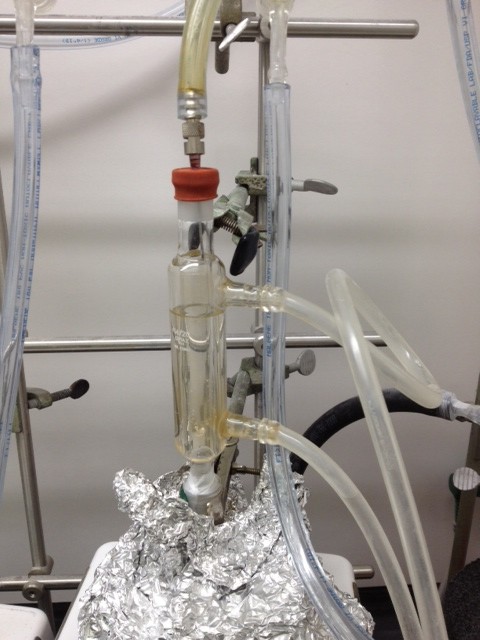

Also, do not just turn off the hotplate/stirrer and go home; if you are unable to begin with the workup right away, you should still wait for at least 30 min to make sure that the reaction mixture is at room temperature and your setup is safe to leave. Below is a picture of what happened when a hotplate was accidentally turned to full heating power rather than to the off position (a simple error in rotating a knob...) and the setup was left unsupervised. It could have ended a lot worse.

- Use the Hood Sashes, and/or a Safety Shield, for any operation that involves vacuum, pressurized vessels, mixing reactive chemicals, or strong acid and bases, to protect you and your lab mates from explosions and exotherms. This real-life example of an evacuated flask clearly also does not meet our minimal safety standards:

- Take Care of Our Equipment: A professional, responsible attitude mandates regular maintenance on personal as well as shared equipment. You cannot expect good performance if you abuse our instruments. Again, pictures say more than a thousand words. The first snapshot is from a pump that has seen a lot of tear and wear, but can still be salvaged. The lower two pictures are from another pump that was operated without a proper cold trap and has suffered a terrible fate in careless hands:
Finally, no laboratory item, especially vacuum pumps, or rotavaps, should be simply "borrowed" or switched from another lab bench!
- Browse: Overview of Lab Techniques and Useful Reagents (TLC, etc) for many useful hints and suggestions!
One of the more obvious and yet most common lapses in experimental technique is the use of septa, needles and syringes in all the wrong places: How good do you think the vacuum is for drying a sample in the left flask, vs the one on the right? Remember, you are in graduate school in Chemistry, and not training for a medical practioner's appointment....


Another major and recurring experimental issue is the presence of gases, particularly oxygen, in solvents for air-sensitive (transition-metal catalyzed, organometallics, etc) reactions as well as chromatography solvents. Air that is plentiful in untreated solvents can interfere in both scenarios and in fact completely prevent a successful operation. Molecular sieves are ineffective in removing residual air. These references provide a good overview of the current techniques, including freeze-pump-thaw, bubbling, and sonication, as well as suggestions for which one to use for what application, and the general background of why degassing is critical for many operations. Please study them carefully (and use the techniques)!